Shielding effect or Screening effect in chemistry:
The reduction in force of attraction of the nucleus on the valence shell due to the inner shell electrons is called the shielding effect. It is also called the screening effect.
The shielding effect depends upon the presence of no. of inner shell and inner shell electrons. As the number of inner shells increases, the shielding effect increases. And if inner shells decrease the shielding effect also decreases.
Let’s try to understand with the help of an example. Consider, you are reading a chemistry lesson in your classroom. And your friends outside the classroom can’t listen to you clearly. Can you tell, me why? This is due to the walls of the classroom, which makes a shield between you and your friend outside. Due to this, your friend would not listen to your voice clearly.
The intensity of your voice heard by your friend outside the room depends on the thickness of the wall. same as the screening effect depends on the number of inner shells. The greater the thickness of the wall (no. of inner shell), the higher will be the shielding effect of the wall. And the smaller the thickness (no. of inner shell), the smaller the shielding effect of the wall.
Factor affecting the screening effect:
The shielding effect depends only on the no. of inner shells. It increases with increasing the inner shell. And decreases with decreasing inner shells.
Shielding effect trends:
Shielding effect Trend down a group:
As we discussed earlier, the screening effect depends on no. of inner shells. The inner shells increase from top to bottom in a group. Therefore, shielding effects also increase down the groups. Look at the example below.
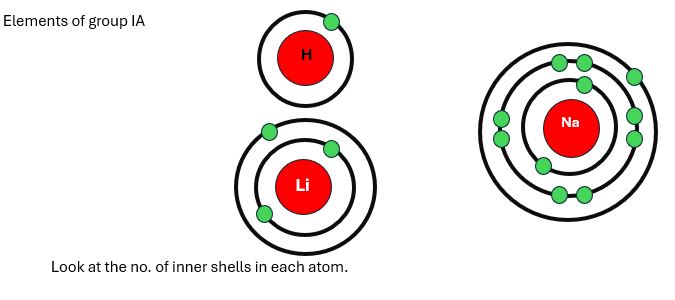 H= No inner shell, Li= One inner shell, Na= Two inner shells
H= No inner shell, Li= One inner shell, Na= Two inner shells
Therefore, Na will have the highest screening effect than Li and H. because Na has the highest number of inner shells. Same as, in the remaining elements of group IA, inner shells increase one by one. So, the shielding effect increases gradually.
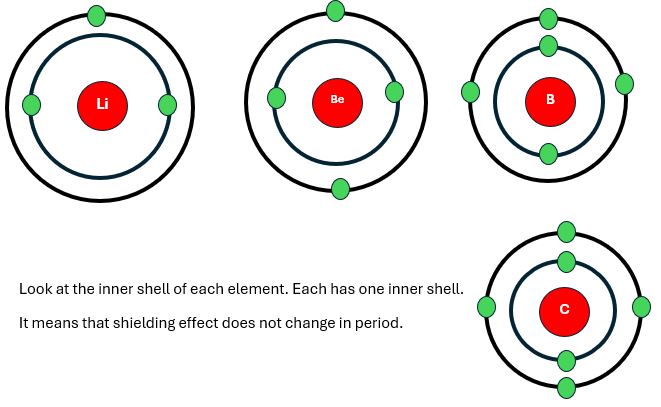
Shielding effect Trend left to right in periods:
The shielding effect in chemistry remains constant across periods. As we discussed earlier that it depends upon the no. of inner shells. And no. of inner shells remains the same from left to right in each period. Therefore, the shielding effect does not increase across the period, it remains constant. For example, consider the elements of period 2.

Pingback: Electron Affinity. What Do You Know About it?
Pingback: Atomic size means in simple words. - thechemistonline789.com Atomic size
Pingback: Understanding of the Metallic And Non-Metallic Character