Electron Affinity:
It is the energy released when an electron adds to a gaseous atom’s valence shell to form a negative ion. The word “affinity” means an attraction for something. So, electron affinity means the attraction of the nucleus for electrons in the valence shell.
M+ e → M-1 + Electron affinity
It is opposite to the ionization energy. And measured in kilojoules per mole. Like ionization energy, it also depends on the atomic size / atomic radii. Greater the atomic radii / atomic size lowers the electron affinity. And the smaller the atomic size / atomic radii, the higher the electron affinity.
For example, in a chemical process, the gaseous fluorine atom gains an electron:
F(g) + e→ F-1 ΔH= -328 kJ/mol
So, the electron affinity of fluorine is -328 kJ/mol. The negative sign shows that it is an exothermic process. while the second electron affinity of the same element is always endothermic because we have to give the energy to force the electron to enter the mono-negative anion.
Electron affinity Trend in the periodic table:
Electron affinity values of elements depend upon the atomic size. It is inversely proportional to the atomic size.
Electron affinity ∞ 1/ atomic size
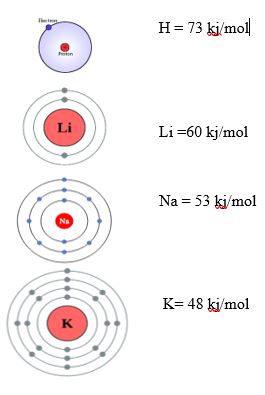
In group:
The electron affinity decreases from top to bottom in a group. Because of the increase in atomic size. As atomic size increases, the attraction of the nucleus on the valence shell decreases. This results in the reduction of attraction of the nucleus to the incoming electron, as a result, electron affinity decreases. For example, consider the group IA of the periodic table.
In period:

The electron affinity increases across the period. Because of the decrease in atomic size. As atomic size decreases in the period from left to right, the attraction of the nucleus on the valence shell for incoming electrons increases. As a result, electron affinity increases. For example, consider the period 2.
Electron affinity exceptions:
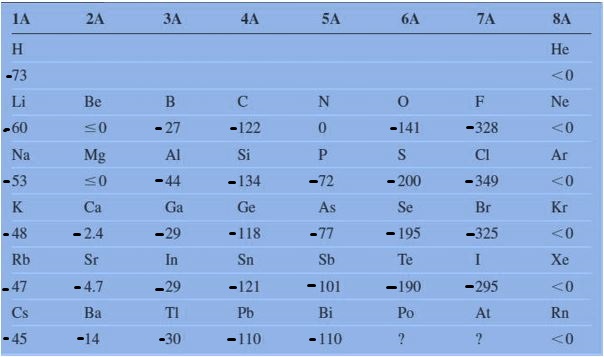
There are some exceptions in electron affinity in the periodic table. Below the table are the electron affinities of representative elements.
Look at the electron affinity values of period 2 and period 3. Where Be, Mg, and N have marked zero electron affinity. To know about the reason behind it, first of all, we have to understand about fully filled orbital, half filled orbital, and partially filled orbitals and their stability.
Fully-filled orbitals (FFO): those orbitals which are filled with electrons. E.g. 3p6, 2s2 etc.
Half-filled orbitals (HFO): Those orbitals that are 50 % filled with electrons. E.g 3p3, 2s1, 3d5 etc.
Partially filled orbitals (PFO): Those orbitals that are filled more than or less than 50 %. E.g. 2p4 and 2p2 both are partially filled orbitals.
Now, the stability order of these orbitals will be as follows.
FFO>HFO>PFO
In the case of Be, Mg, and N. Be and Mg have completely filled orbital 2s2 and 3s2. Therefore, it is difficult to add an electron into its valence shell. And instead of releasing energy, we need to give energy to add the electron. Due to this reason, the electron affinity of both Be and Mg are marked as zero, sometime it may also have a positive value..
Same as in the case of N, it has a half-filled 2p3 orbital that is also somehow stable. Therefore its electron affinity value is also marked as zero or sometimes its value is marked as +7. And the electron affinity values of noble gases are also marked as zero. Because we know well that noble gases have completely-filled valence shells therefore they don’t accept electrons in normal conditions.
Factors affecting the electron affinity:
The electron affinity of an element is affected by two factors.
- Electronegativity
- Atomic size
- Shielding effect
Electronegativity ∞ Electron affinity:
The higher the electronegativity, the higher the electron affinity. And lower the electronegativity and lower the electron affinity.
Atomic size∞ 1/ Electron affinity :
The larger the atomic size, the lower the electron affinity. And the smaller the atomic size, the higher the electron affinity.
Shielding effect ∞ 1/ Electron affinity:
if the shielding effect is high then the electron affinity becomes low. while if the shielding effect is low then the electron affinity becomes high.

Thanks for finally writing about >Electron Affinity.
What Do You Know About it? <Loved it! https://Www.Waste-Ndc.pro/community/profile/tressa79906983/
Thanks foor finally writing about >Electron Affinity. What Do You Know About it?
<Loved it! https://Www.Waste-Ndc.pro/community/profile/tressa79906983/