Metallic Character:
Metallic character is defined as the property of an element to lose an electron from its valence shell called metallic character. Of all the elements in the periodic table, only the metal family has the character to lose electrons. Therefore, metallic character is associated with metals.
Factors affecting metallic character:
Metallic character is affected by
Atomic size / Atomic radii:
The metallic character is proportional to the atomic size. The larger the atomic size, the higher the metallic character. Because of its large atomic size, the nucleus loosely holds the valence electron and it is easy to lose the electron and vice versa.
Shielding effect / Screening effect:
The metallic character is also directly proportional to the shielding effect/screening effect. The higher the shielding or screening effect, the higher the metallic character vice versa. Because due to the higher shielding effect, the nucleus loosely holds the valence electron, and it becomes easy to lose valence electrons.
Electronegativity:
Metallic character is inversely proportional to the electronegativity. If the electronegativity is high, then the metallic character becomes low. If the electronegativity is low, then the metallic character becomes high.
Metallic character in periodic table:
the metallic character vary both in a group and period of the periodic table.
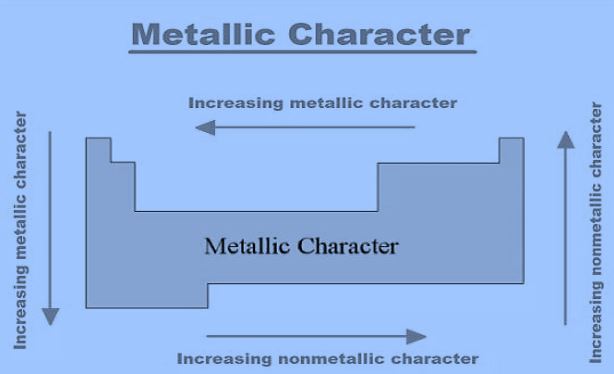
The metallic character increases from top to bottom. Because as we have discussed earlier, it depends upon atomic size and shielding effect. Therefore, in a group both the shielding effect and atomic size increase in a group due to which attraction of the nucleus on the valence shell decreases as a result metallic character increases in a group.
Metallic character, periodic trend in period:
Metallic character decreases from left to right in a period. Because atomic size decreases from left to right, it increases the force of attraction of the nucleus on the valence shell. Therefore, it becomes difficult for an element to lose its electron from the valence shell.
Non-metallic character:
Non-metallic character is opposite to the metallic character. It can be defined as the ability of an atom to attract an electron towards itself called non-metallic character. In the periodic table, non-metal elements show this property. Therefore, non-metallic character is associated with non-metals.
Factors affecting non-metallic character:
Three factors influence non-metallic character.
- Atomic size / Atomic radii
- Shielding effect / Screening effect
- Electronegativity
Non-metallic character ∞ 1/Atomic size or atomic radii:
Non-metallic character is inversely proportional to the atomic size or radii. The larger the atomic size or radii, the smaller the non-metallic character, and vice versa. Because, if the atomic size is large then the attraction of the nucleus on the valence shell becomes weak and it becomes difficult to gain an electron for an atom. While, if the atomic size or radii is small then the attraction of the nucleus on the valence shell becomes high as a result non-metallic character increases.
Non-metallic character ∞ 1/Shielding effect or Screening effect:
Non-metallic character is also inversely proportional to the shielding or screening effect. The higher the shielding or screening effect, the smaller the non-metallic character, and vice versa. Because, if the shielding or screening effect is high then the attraction of the nucleus on the valence shell becomes decreases therefore non-metallic character decreases. While, if the shielding or screening effect is less then the attraction of the nucleus on the valence shell is high therefore non-metallic character increases.
Non-metallic character ∞ Electronegativity:
The non-metallic character also depends upon the electronegativity of an atom. If the electronegativity is high, then the non-metallic character becomes also high. If the electronegativity is low, then the non-metallic character becomes also low.
Non-metallic characters in periodic table:
It also varies both period and group of the periodic table.
Trend in Group:
The non-metallic character decreases down the group. Because both atomic size and shielding effects increase from top to bottom.
Trend in period:
Non-metallic character increases across a period from left to right. Because atomic size decreases from left to right which is responsible for the increase in non-metallic character.
(FAQs)
-
Why does metallic character increase down the group?
Because in a group both the shielding effect and atomic size increase down the group. Due to this attraction of nucleus on the valence shell becomes weak. Therefore, it becomes easy to lose an electron in an atom from its valence shell.
-
What is the definition of a non-metallic character?
Non-metallic character is the ability of an atom to attract an electron towards itself called non-metallic character. This is the property of non-metals.
-
What is the characteristics of metallic elements?
A metallic element loses electrons from its valence shell. The more easily a metal loses electrons more will be the metallic character and vice versa.
-
What is the metallic character pattern?
The metallic character pattern is that it increases in a group and decreases across a period.
-
What defines metallic character?
Metallic character defines the ability of an atom to lose electrons from its valence shell.
-
What are the 3 metallic characters?
The three metallic characters are as follows
- They easily lose an electron
- Good conductor of heat and electricity
- Have a shiny surface.
-
Why non-metallic character decreases down the group?
Because of the increase in atomic size and shielding effect, non-metallic character decreases down the group.
-
Why non-metallic character increases across a period?
Because of the decrease in atomic size, it increases across the period.
-
Difference between metallic and non-metallic character.
| Metallic Character | Non-metallic Character |
|