Reactivity of Elements:
reactivity of elements is defined in chemistry as the ability of an element to gain or lose electrons. It depends upon the nature of an element, whether metal or non-metal. The reactivity of metals depends upon the ability to lose electrons easily while non-metals depend upon the ability to gain electrons easily.
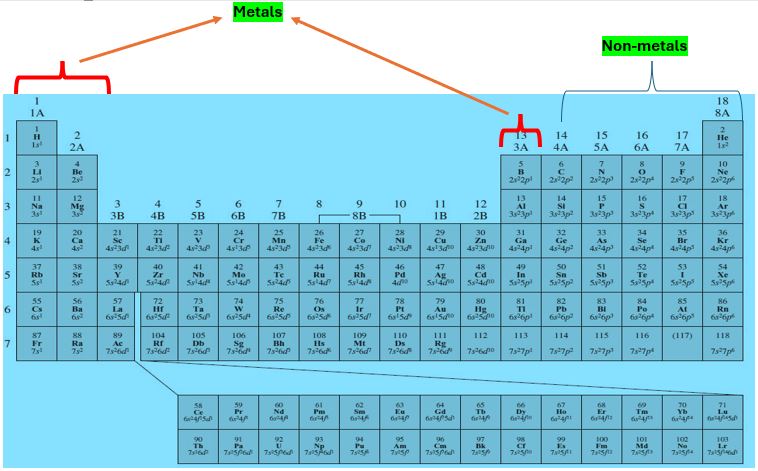
Before discussing the reactivity of elements in the periodic table, we try to know the position of metals and non-metals in the periodic table. So, in the periodic table, the majority of elements in groups 1 (IA),2 (IIA), and group 13 (IIIA) are metals. While the majority of elements in groups 14 to 18 (IV A to VIII A) are non-metals. We separately discuss the reactivity of metal and non-metal.
Reactivity of metals:
The reactivity of elements (metals) is defined as the ability of a metal to lose electrons easily from its valence shell called reactivity of metal. It is related to the metallic character. The higher the metallic character, the higher the reactivity of the metal. The lower the metallic character, the lower the reactivity.
Factors affecting the metal’s reactivity:
The following are the factors that affect the reactivity of the metal.
Atomic size or radius ∞ Reactivity: Metal’s reactivity is directly proportional to the atomic size or radius. The larger the atomic size, the higher the reactivity of metal and vice versa.
Shielding or screening effect ∞ Reactivity: Metal’s reactivity is also proportional to the shielding or screening effect. The higher the shielding effect, the higher the reactivity of metal and vice versa.
Electronegativity ∞ 1/Reactivity: The reactivity of elements (metal) is inversely proportional to the electronegativity. The higher the electronegativity, the lower the reactivity of the metal. While the lower the electronegativity, the higher the metal’s reactivity.
Reactivity on Periodic table:
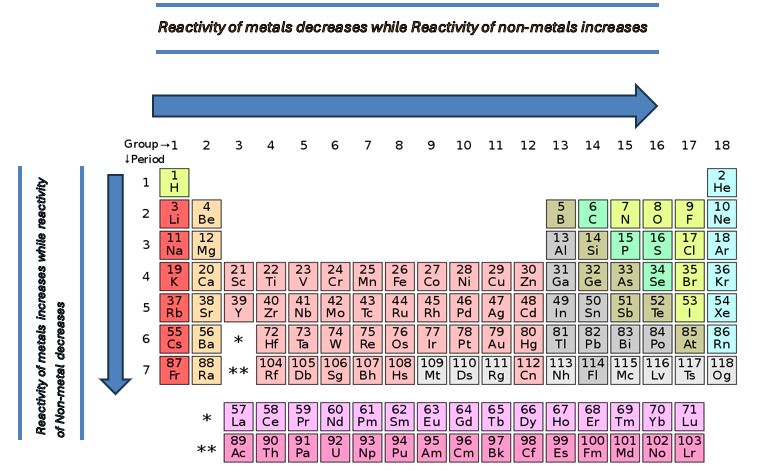
The reactivity of metals varies both in the period and group of the periodic table.
Reactivity trend of metals in Group: The reactivity of metals increases in a group from top to bottom because of an increase in atomic size and shielding effect and a decrease of electronegativity.
e.g Reactivity of elements in group 1
H<Li<Na<K<Rb<Cs<Fr
Reactivity series of group 2
Be<Mg<Ca<Sr<Ba<Ra
Reactivity Series of Group 3
B<Al<Ga<In<TI
Reactivity trend of metals in Period: In period, the reactivity of metals decreases from left to right due to a decrease in atomic size and increase of electronegativity, while as we know well the shielding effect remains constant in the period therefore it has no effect on reactivity in period.
For example, the Reactivity of period 2
Li> Be> B
Reactivity of non-metals:
It is defined as the ability of non-metals to gain electrons easily. Because non-metals always gain electrons. Therefore, their reactivity depends on the ability to gain electrons. Easily a non-metal gain electron, the higher the reactivity of non-metal. While more difficult for a non-metal to gain electrons, less the reactivity.
Factors affecting the reactivity of non-metals:
It also depends upon the following factors.
- Atomic size
- Electronegativity
- Shielding effects
Reactivity of non-metal ∞ 1/Atomic size:
The larger the atomic size, the lesser the reactivity of non-metal. While the smaller the atomic size, the higher the reactivity.
Reactivity of non-metal ∞ Electronegativity: If the electronegativity is high then the reactivity will be also high. While if the electronegativity is low then the reactivity will also be low.
Reactivity of non-metal ∞ 1/Shielding effect: The higher the shielding effect, the lower the reactivity of non-metal. While the lower the shielding effect, the higher the reactivity.
Reactivity trend for non-metals:
It also changes both the period and group of the periodic table depending on the atomic size, electronegativity, and shielding effect. We discuss the trend in both group and period.
Trend in Group: The reactivity of non-metals decreases down the group because, as we have discussed earlier it depends upon the atomic size, electronegativity, and shielding effect. Therefore, the shielding effect and the atomic size increase in a group while the electronegativity decreases. All these three factors reduce the attraction of the nucleus on the valence shell thus the reactivity of non-metals decreases in a group.
Trend in Period: In period, reactivity increases from left to right because of a decrease in atomic size and an increase in electronegativity. These two factors increase the attraction of the nucleus on the valence shell. Thus, the reactivity of non-metals increases.
In short, the reactivity of elements in the periodic table is different. It depends upon the nature of elements whether they are metal or non-metal. The reactivity of metals increases in the group and decreases in the period. While the reactivity of non-metal elements decreases in the group and increases in the period.
Frequently Asked Questions (FAQs)
- Which is the most reactive metal Class 10 answer?
In the whole periodic table, Francium (Fr) is the most reactive metal due to its large atomic size and high shielding effect.
- Which is the most reactive non-metal class 10?
The most reactive non-metal in the whole periodic table is Fluorine (F). which is due to the smallest atomic size.
- Which is the most reactive metal in the first 20 elements?
From the first 20 elements of the periodic table, the most reactive metal is Potassium (K) due to its large atomic size and low ionization energy.
- Which is the least reactive element?
Helium (He) is the least reactive element in the whole periodic table, due to its stable electronic configuration.
- What is the second most reactive metal?
The second most reactive metal in the periodic table is the Cesium (Cs) metal.
- What are the top 4 most reactive metals?
The top 4 most reactive metals are Francium (Fr), Cesium (Cs), Rubidium (Rb), and Potassium (K).
- Which metal family is the most reactive?
Group 1 (IA) / Alkali metals are the most reactive metal family in the periodic table due to their larger atomic size than the remaining groups of the periodic table.
- Which nonmetal family is the most reactive?
Group 17 (VII A) / Halogen family is the most reactive non-metal family in the periodic table due to its smaller atomic size.
- Which metal is liquid at room temperature?
Mercury is a metal which is liquid at room temperature.
- What are the least reactive metals?
Gold, Silver, and Platinum are the least reactive metals.