What is Atomic size / atomic radii
Atomic size means the average distance between the nucleus and the valence shell of an atom is called atomic size / atomic radii. The greater the distance between the nucleus and the valence shell, greater will be the atomic size. The smaller the distance between the nucleus and the valence shell, the smaller will be the atomic size.
Factors affecting the atomic size / atomic radii:
The atomic size depends upon the following two factors.
- No. of shells
- Shielding effect
Atomic size ∞ no. of shells: Atomic size is directly proportional to the no. of shells. As the number of shells increases in an atom. The distance between the nucleus and the valence increases. Therefore, it increases with increasing the no. of the shell. And as the no. of shell decreases. The distance between the nucleus and the valence shell also decreases. Therefore, atomic radii decrease.
Atomic size ∞ shielding effect: Atomic size is also directly proportional to the shielding effect. The greater the shielding effect larger the atomic size and decreasing the shielding effect. Smaller the atomic size.
Atomic Size Trend in Periodic Table:
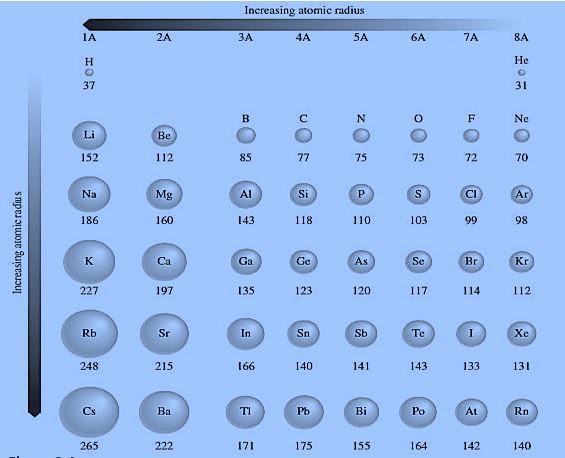
Atomic size varies both in period and group. First, we discuss the trend in a group.
Trend In Group:
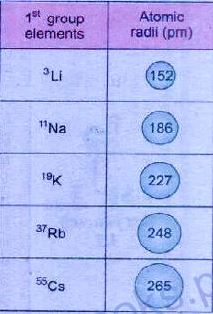
In a group, atomic size increases from top to bottom. It is due to an increase in the
and no. of shells. For example, consider group IA.
In the first group, Lithium has atomic size 152 pm, Sodium has 186 pm, Potassium has 227 pm, Rubidium has 248, and Caesium has 265 pm. This shows that atomic size is increasing from top to bottom.
Trend In Period:
In a period, atomic size decreases from left to right. Now, in this case, the reason is not the no. of shells and shielding effect. Because both no. of shells and the shielding effect remain constant across the period. The reason is that the effective nuclear charge and the no. of valence electrons increase one by one from element to element across each period. Which results in a gradual increase in the force of attraction between the nucleus and the valence shell. Therefore, atomic size decreases across the period. For example, periods 1 to 3.
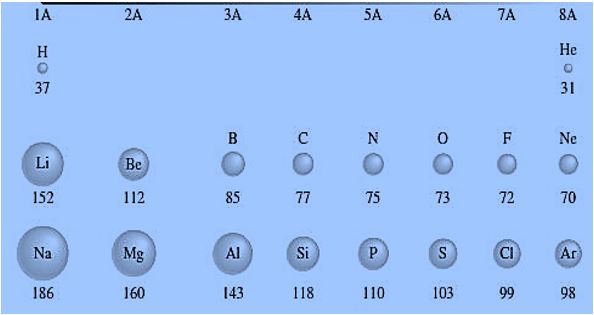
In period 1, the atomic size of Hydrogen (H) is 37 pm, and Helium (He) is 31. In 2nd period, Lithium (Li) has an atomic size of 152 pm, Beryllium (Be) has 112 pm, Boron (B) has 85 pm, Carbon (C) has 77, Nitrogen (N) has 75 pm, Oxygen (O) has 73 pm, Fluorine (F) has 72 and Neon (Ne) has 70. While in 3rd period, Na has an atomic size of 186 pm, Mg has 160 pm, Al has 143, Si has 118, Phosphorous (P) has 110 pm, Sulphur (S) has 103 pm, Chlorine (Cl) has 99 pm and Argon (Ar) has 98 pm. In all the above periods, atomic size increases from left to right.
The atomic size chart of representative elements is given below. Which makes your concepts about trends in both periods and groups clear.
Unit of atomic radii / atomic size:
The unit of atomic radius is a picometer (10-12 m). So, it is measured in a picometer.
How to find atomic radius / atomic size?
There are many techniques and empirical formulas are available to find the atomic radius. These are given below:
Empirical formulas:
- Schomaker and Stevenson’s formula:
r= (2.5-0.1Z)/(n2)
r= Atomic radius in Ao
Z= Atomic number
n= no. of electrons in the outermost shell
- Goldschmidt’s formula:
r= (1.44/Z+1) n2
r= Atomic radius in Ao
Z= Atomic number
n= no. of electrons in the outermost shell
- Zeff formula:
r= (1.38/Zeff2)
r= Atomic radius in Ao
Zeff= effective nuclear charge
- Slater’s rule:
r=(1.2/Z-1.4)
r= Atomic radius in Ao
Z= Atomic number
- Allred-Rochow’s formula:
r=(0.98/Z+1.2)
r= Atomic radius in Ao
Z= Atomic number
Techniques to find atomic radius:
The following techniques can be used to find the atomic radius.
- X-ray diffraction techniques
- Electron spin resonance (ESR) Spectroscopy
- Nuclear magnetic resonance (NMR) Spectroscopy
- Quantum Mechanical Calculations like Density Functional Theory (DFT).


2 Replies to “Atomic size means in simple words.”