Electronegativity is a measure of an atom’s ability to attract an electron pair toward itself in a chemical bond to form an anion called electronegativity. In the periodic table, the most electronegative elements are halogen elements, while the least electronegative elements are alkali metals.
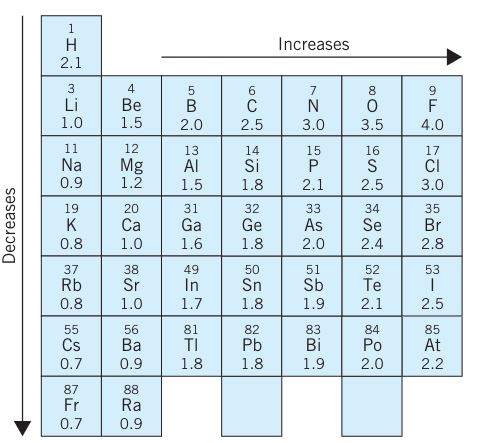
The electronegativity polarity scale was first time introduced by the American chemist Linus Pauling. The electronegativity Pauling scale for the elements is given below:
The electronegativity chart shows that electronegativity decreases down the group and increases across the period.
Factors affecting the electronegativity:
The following are the factors that affect the electronegativity of an element.
- Atomic size / Atomic radii
- Effective nuclear charge
The greater the atomic size / atomic radii, the smaller will be the electronegativity. As atomic size increases the attraction of the nucleus on the valence shell decreases as a result electronegativity becomes less.
While the smaller the atomic size / atomic radii, the stronger the electronegativity. As atomic radii decrease the force of attraction of the nucleus on the valence shell increases as a result electronegativity becomes high.
The effective nuclear charge also affects the electronegativity. The greater the effective nuclear charge, the stronger the attraction of the nucleus on the valence shell, and the electronegativity will be high.
And lesser the effective nuclear charge, the weaker the attraction of the nucleus on the valence shell, as a result, electronegativity will be less. The electronegativity values change both in period and group.
Trend In Groups and Periods:
Electronegativity in the periodic table varies in both periods and groups.
Electronegativity trend in the group:
Electronegativity in elements of groups decreases from top to bottom. Because atomic size increases from top to bottom. As a result force of attraction of the nucleus on the valence shell decreases. Therefore, electronegativity decreases down the group.
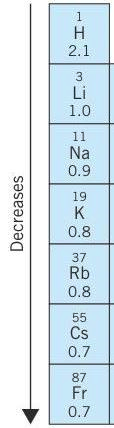 Electronegativity examples in group
Electronegativity examples in group
Electronegativity trend in period:
In period, electronegativity increases across the period. Because in period effective nuclear charge increases one by one from one to another atom. Which increases the force of attraction between the nucleus and valence shell therefore electronegativity increases in period.
For example, electronegativity across period 3 is given below.
Electronegativity of Na (Sodium) is 0.9, Mg has 1.2, Al has 1.5, Si has 1.8, P has 2.1, electronegativity of Sulfur is 2.5, and electronegativity of Cl (Chlorine) is 3. These values show that electronegativity increases across periods.
Importance of electronegativity:
The electronegativity difference between the bonded atoms finds the nature of a covalent bond. The electronegativity difference for polar bonds is 1.7. if the difference is greater than 1.7 then the bond will be a pre-dominantly polar covalent bond. If the electronegativity difference between the bonded atoms is less than 1.7 the bond will be pre-dominantly non-polar.
By using electronegativity values, we can also predict, whether the bond is ionic or covalent. when the bond forms between the high electronegative atoms (Halogens) and a low electronegative atom (alkali metals), then the complete transfer of electrons takes place between them as a result the bond will be ionic.
2Na (Alkali metal) + Cl2(Halogen)🡪 2NaCl (Ionic compound)
It also affects the ionization energy of elements. the greater the electronegativity, the higher will be the ionization energy. while the lesser the electronegativity, the lower will be the ionization energy.


Pingback: Electron Affinity. What Do You Know About?
Pingback: Understanding of the Metallic And Non-Metallic Character