What is Ionization energy:
Ionization/ionisation energy meaning that the minimum amount of energy in Kj/mol required to remove an electron from the valence shell of an isolated gaseous atom to form a cation is called ionization energy.
In other words, ionization energy is defined as,the amount of energy in kilojoules needed to expel 1 mole of electrons from the valence shell of a 1-mole gaseous atom to form 1 mole of cations. Ionization energy in chemistry determines how tightly an atom’s nucleus holds electrons.
A (g) + Energy → A+1
The stronger the attraction between electrons and nuclei, the higher the ionization energy will be; the weaker the attraction between electrons and nuclei, the lower the energy will be.
Example of ionisation energy:
the Ionization energy of Hydrogen is 1,312 Kj/mol
H + Energy →H+1 + e
While the ionization energy value of Lithium is 520 Kj/mol
Li + Energy →Li+1 + e
The ionisation energy values of Hydrogen and Lithium show that the nucleus more strongly holds the valence electron of Hydrogen than the nucleus of Lithium.
What is the first ionization energy?
First ionization energy is the energy required to remove the first electron from the valence shell of the gaseous atom.
e.g. the ionization energy of Hydrogen and Lithium discussed above is the first ionization energy value of both.
What is the second ionization energy?
The second ionization energy is the amount of energy required to remove the second electron from the unipositive gaseous atom. The ionization energy 2nd of Lithium is 7300 kJ/mol.
Li+1 + Energy → Li+2 + e
What is the third ionisation energy?
And the third ionisation energy is the amount of energy needed to remove the third electron from a dipositive cation.
E.g the third ionisation energy of Lithium is 11,815 kJkJ/mol.
Li+2 + Energy→ Li+3 + e
The second and the third ionization energy of any atom is always higher than the first ionization energy as in the case of lithium. This is because as we remove the first electron, the force of attraction of the nucleus on the remaining electrons becomes stronger and stronger. Therefore, the second and third ionization energy is higher than the first. The order of ionization energy 1st, 2nd, 3rd are given below.
1st ionization energy< 2nd ionization energy< 3rd Ionization energy
What do you think? Ionization energy is the endothermic process or exothermic process? The answer is that the ionization energy is always an endothermic process. As we know in ionization energy an atoms or ions absorb energy during ionization and always have a positive value.
Ionisation energy Trend:
Ionization energy varies both in group and period. It has a different trend.
Ionization energy in group:
In a group, ionisation energy decreases down the group. Because as we go down the group, the number of shells or shielding effects increases down the group. As a result, the attraction of the nucleus on the valence electrons decreases. Therefore ionization energy decreases.
The ionization energy numbers of group 1A are given below.
Ionisation Energy Symbol (E) H = 1312 Kj/mol
Ionisation Energy Highest to Lowest 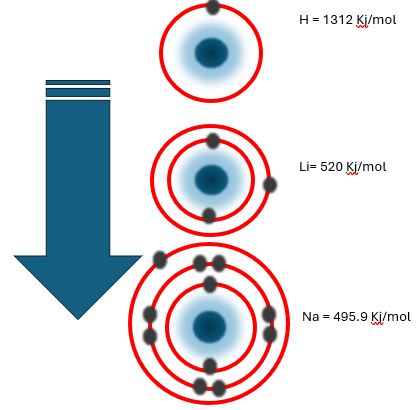
Same as in group 1, ionisation energy groups 2, 3,4, 5, etc also decrease.
Ionization energy left to right in period:
In period, ionization energy increases from left to right. Because in the period nuclear charge increases from one atom to another atom one by one. As a result attraction between the nucleus and valence shell increases. Therefore ionization energy increases across the period.
e.g Ionisation energy period 1 H= 1312 Kj/mol, He = 2373 kj/mol
The ionization energy of Helium is higher than the ionization energy of Hydrogen.
Ionisation energy period 2 Li = 520 Kj/mol, Be= 899 Kj/mol, B= 801 Kj/mol,
C= 1086 Kj/mol, N= 1400 Kj/mol, O= 1314 Kj/mol
Ionization energy trend period 3
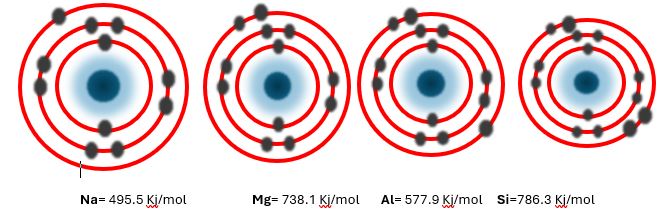
Factors affecting the ionisation energy:
Ionisation energy depends on the following factors:
- Atomic size / Atomic radii
- Shielding effects
The greater the atomic size / atomic radii. The weaker the force of attraction between the nucleus and the valence shell electrons. Hence, ionisation energy will be low.
While, the smaller the atomic size / atomic radii. The stronger will be the force of attraction between the nucleus. Hence, ionisation energy will be high.
Ionisation energy chart or Ionization energy table
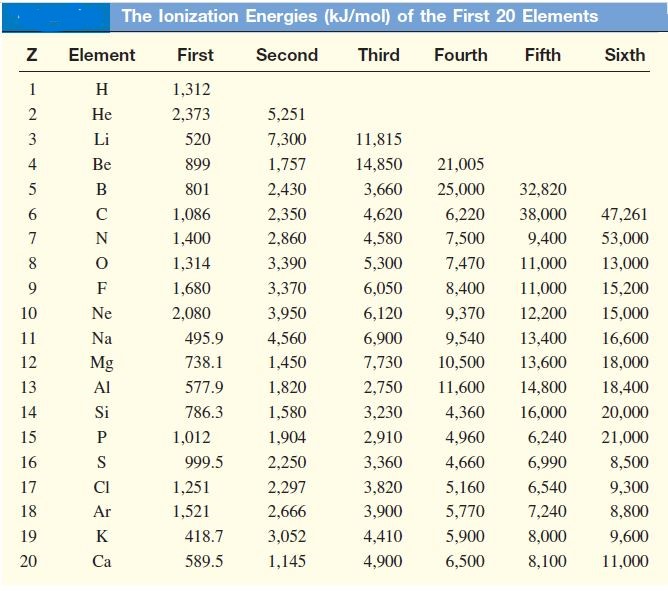
Ionisation energy exceptions:
The ionisation values of S and P-block elements are given below.
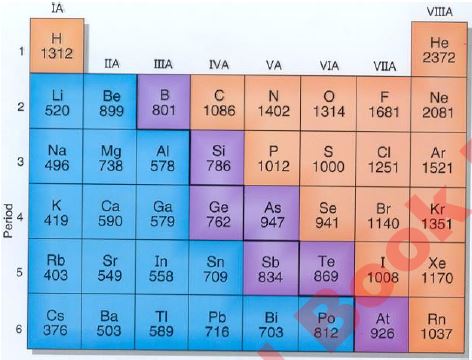
Generally, ionisation energy values across a period increase from left to right but there are some abnormalities in the period. Look at period 1, ionisation energy for Hydrogen atoms is 1312 Kj/mol. And ionization energy of Helium is 2372 Kj/mol. Which is according to the trend.
The ionization energy period 2 and ionization energy period 3 and in other periods are also according to the general trend. But ionisation energy of group 2 elements and ionization energy group 5 (N, P, and As) are not follow the general trend. In period 2, the ionization energy Lithium is 520 Kj/mol, the ionization energy beryllium is 899 Kj/mol. The ionization energy boron is 801 Kj/mol, the ionization energy carbon is 1086 Kj/mol. The ionization energy nitrogen is 1402 Kj/mol and ionization energy oxygen is 1314kj/mol.
Abnormal trends in ionization energy across period 2:
All the elements in period 2 are following the general trend except beryllium and Nitrogen. This is because beryllium has a stable filled 2s orbital than the unstable partially filled 2p orbital of Boron. Same as, Nitrogen has a stable half-filled 2p orbital than the unstable partially filled 2p orbital of Oxygen. And it is difficult to remove an electron from a stable orbital. Therefore, Beryllium and Nitrogen both have high ionization energy and show abnormal trends.
Ionisation energy period 3:
While ionisation energy period 3, the ionization energy of sodium is 495 kj/mol, ionisation energy of magnesium is 738 kj/mol. The ionization energy for aluminum is 578 kj/mol, the ionization energy silicon is 786 kj/mol. The ionization energy for phosphorous is 1012 kj/mol, the ionization energy sulfur is 1000 kj/mol, ionization energy of chlorine is 1251 kj/mol. All these elements follow the general trend except the Magnesium and Phosphorous. They have the same reason as in the case of Beryllium and Nitrogen.
Ionization energy, how to find?
This is the question about the ionisation energy calculation that arises in our mind. The ionisation energy equation used for the calculation is known as the Rydberg Equation which is given below:
ΔEi=A(1/nf2 – 1/ni2)
Where, A= 2.18×10-18 joules, nf=∞, ni= Starting energy level
The above Rydberg equation is the ionisation energy formula used to calculate the ionisation energy values in the periodic table.

Good explanation
Assalamu Alaikum, the article you have written ionization, and what you have explained on energy is very logical.
You have perfected what you have explained on ionization energy and it is very helpful for us.
Perfect explanation
👍
It’s very important information, thank you.
Hey Students!
Summer semester’s almost here! Get a head start and grab all your eTextbooks (over 15,000 titles in convenient PDF format!) at Cheapest Book Store. Save BIG on your studies with 20% off using code SUMMERVIBE24.
Still missing a book? No problem! Submit a request through our system and we’ll add it to our collection within 30 minutes. That’s right, you won’t be left scrambling for materials! ⏱️
Don’t wait – visit https://m.cheapestbookstore.com today and ace your summer semester!
Happy Learning!
Cheapest Book Store
Pingback: #What is electronegativity? - thechemistonline789.com
I know a lot of folks whom I think would really enjoy your content that covers in depth. I just hope you wouldn’t mind if I share your blog to our community. Thanks, and feel free to surf my website Webemail24 for content about Social Media Marketing.
No issue bro. But give me a backlink.